Alkene oxidation to geminal-diol (cis) by cold, dilute, alkaline Potassium Permanganate (KMnO4). - YouTube
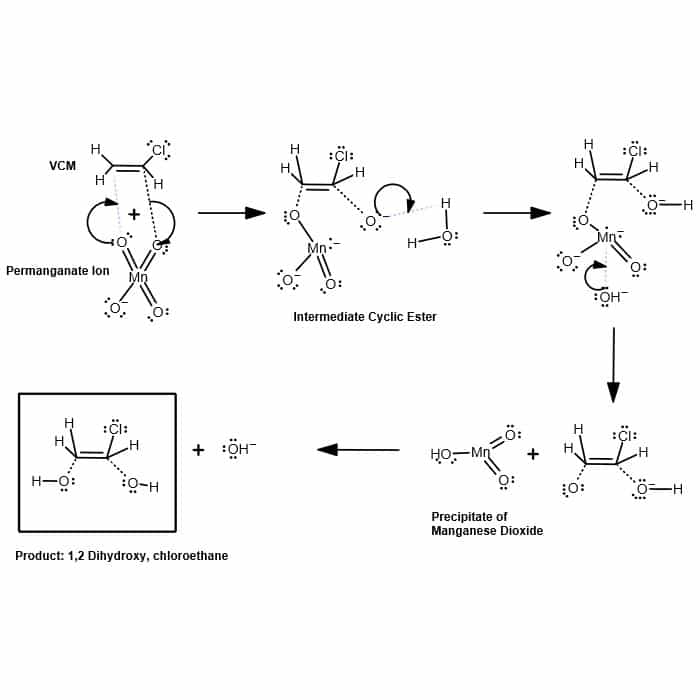
Reaction For Removal of Vinyl Chloride Using Potassium Permanganate | HS-600 | Hydrosil International
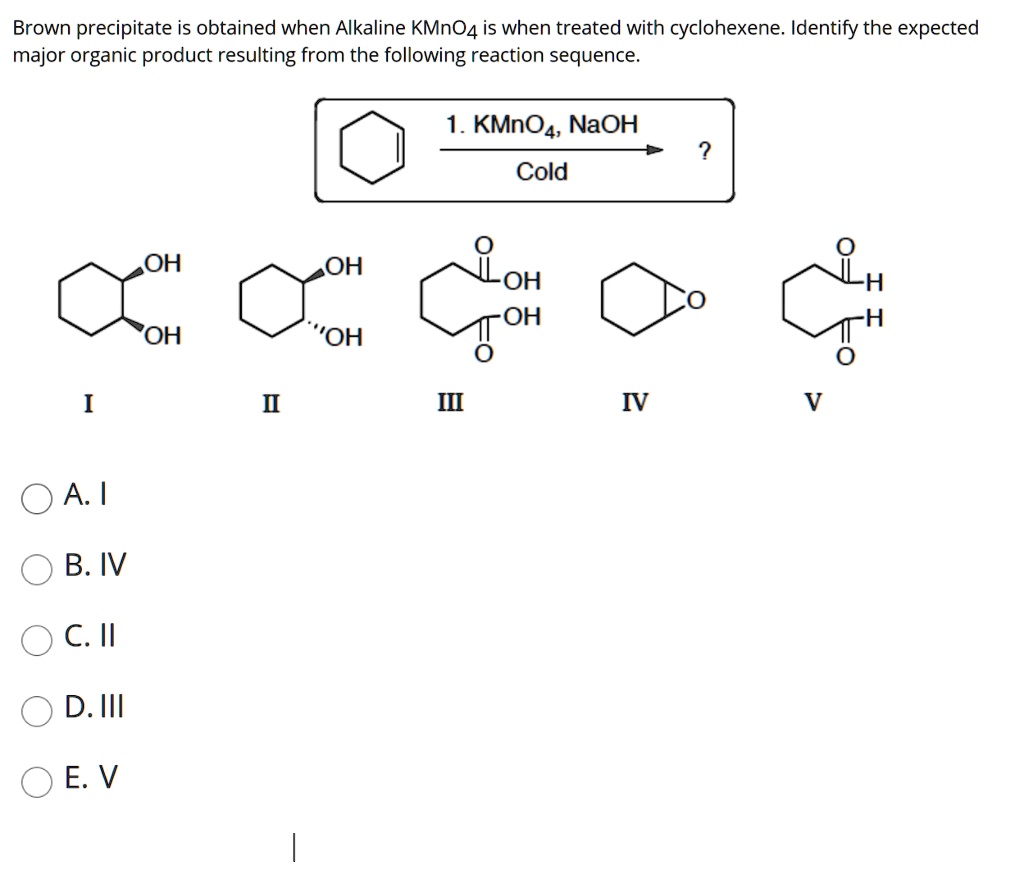
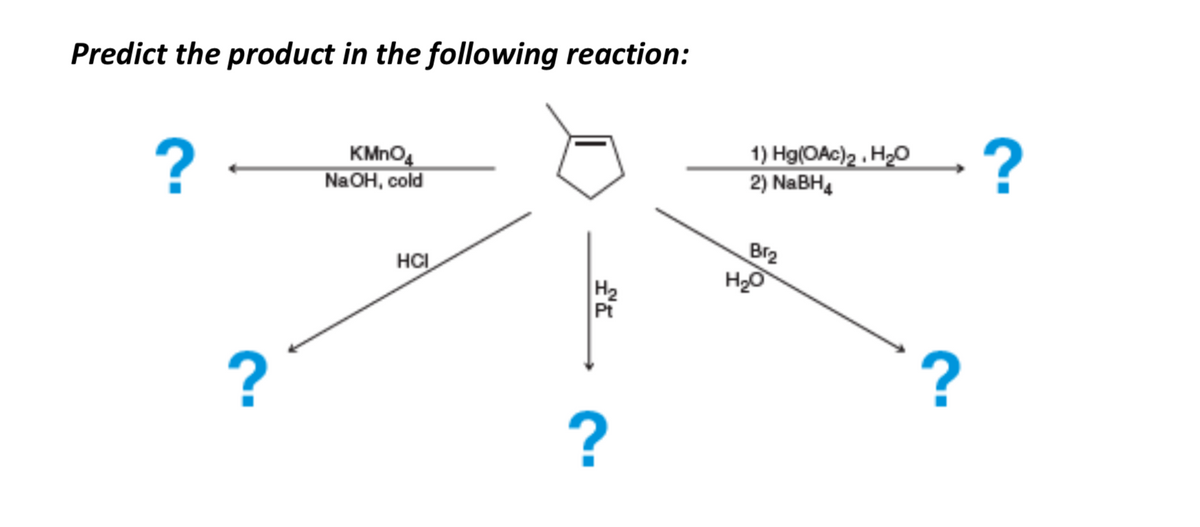
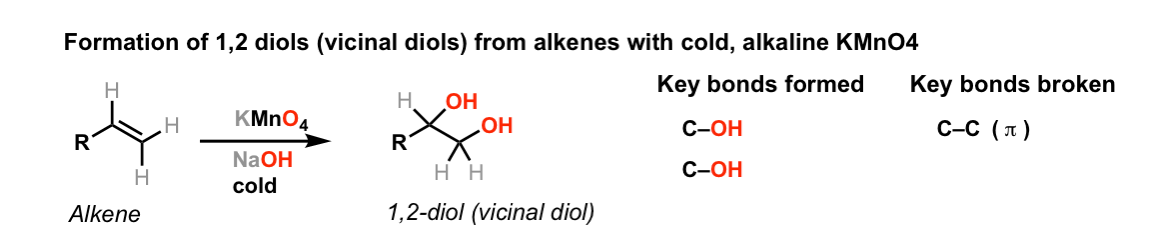
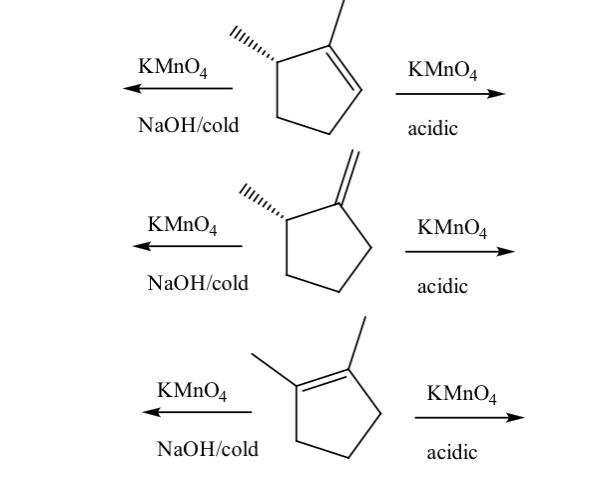
SOLVED: Brown precipitate is obtained when Alkaline KMnO4 is when treated with cyclohexene. Identify the expected major organic product resulting from the following reaction sequence. 1 KMnO4, NaOH Cold OH OH OH

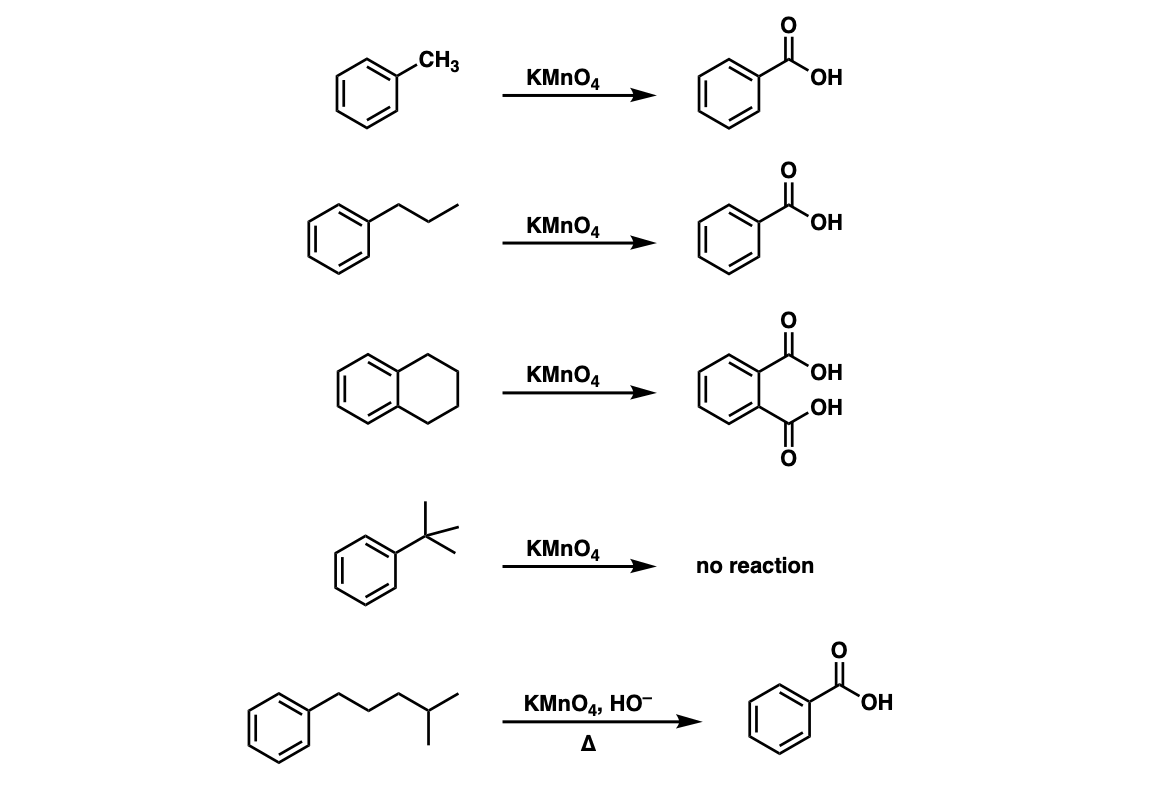

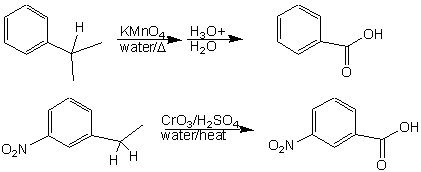

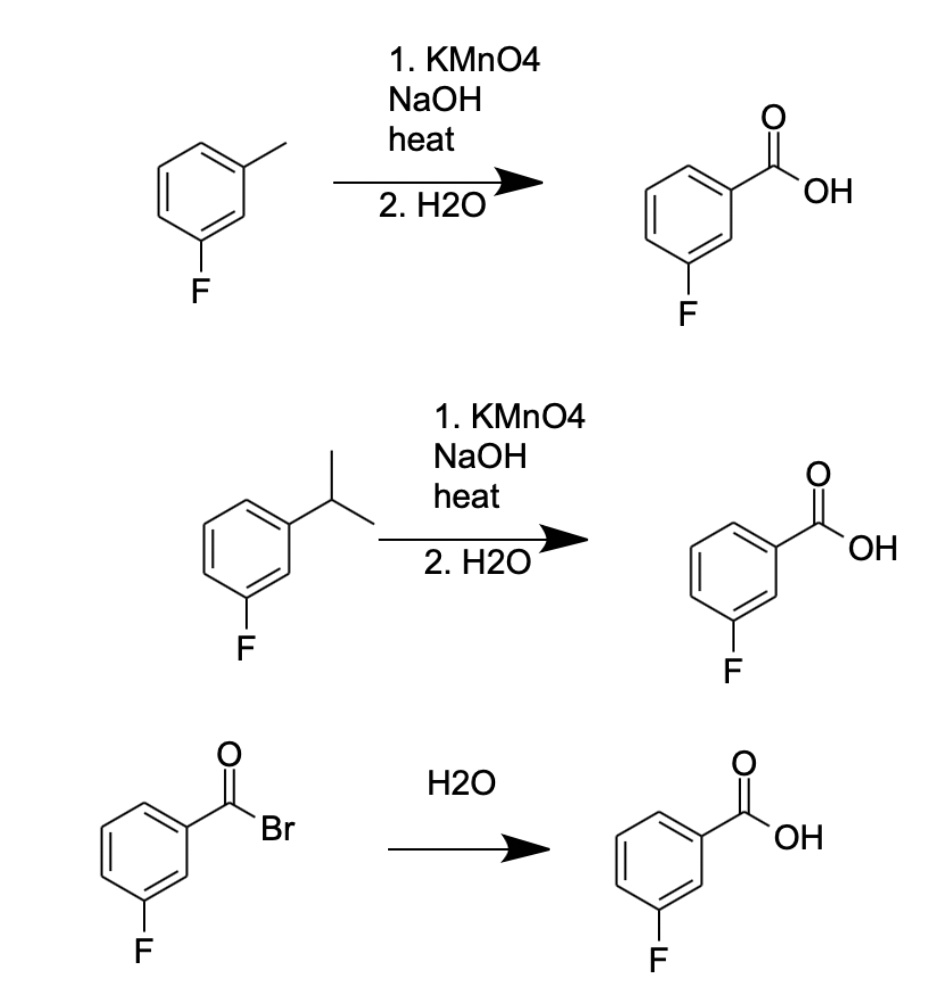
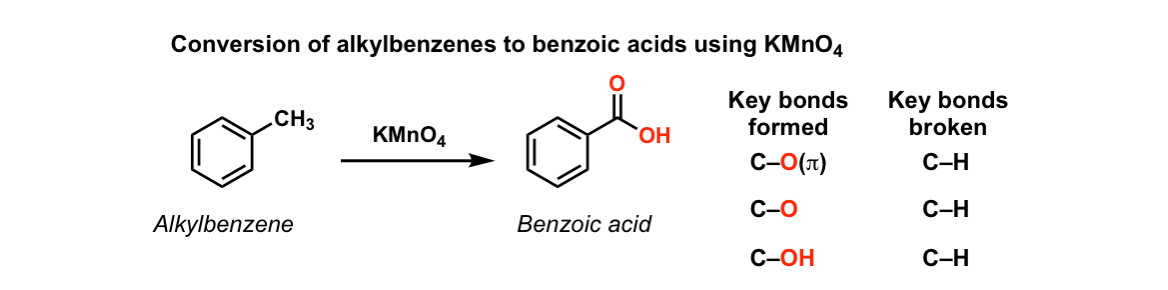
![Oxidation to carboxylic acid [H2CrO4 or KMnO4] - ChemistryScore Oxidation to carboxylic acid [H2CrO4 or KMnO4] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Oxidation-to-carboxylic-acid-H2CrO4-or-KMnO44-768x316.png)