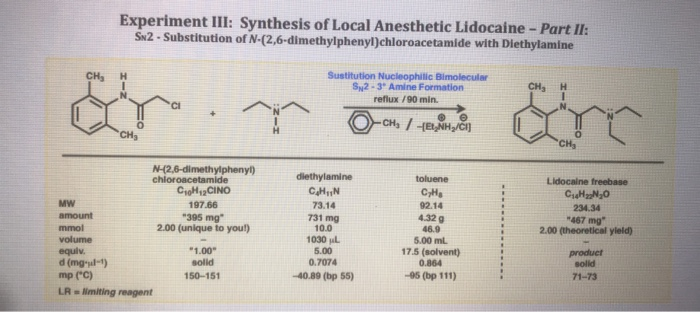
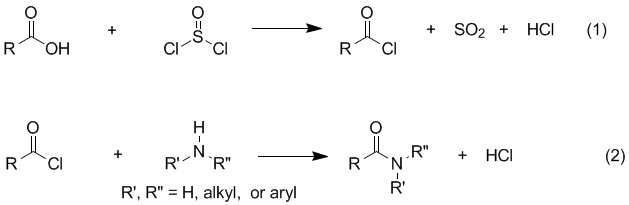
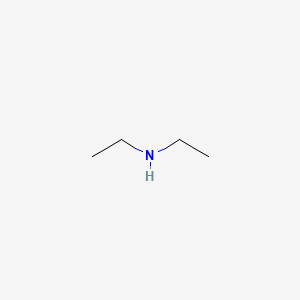
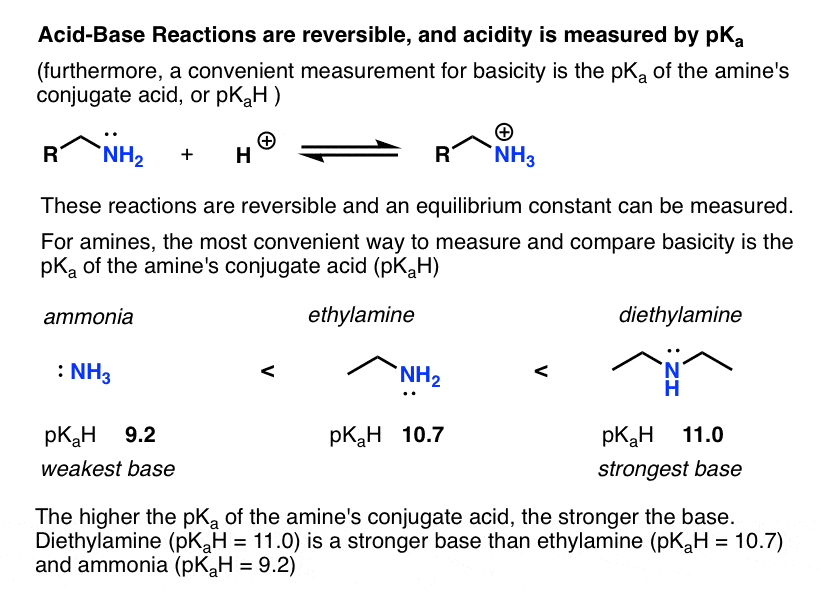
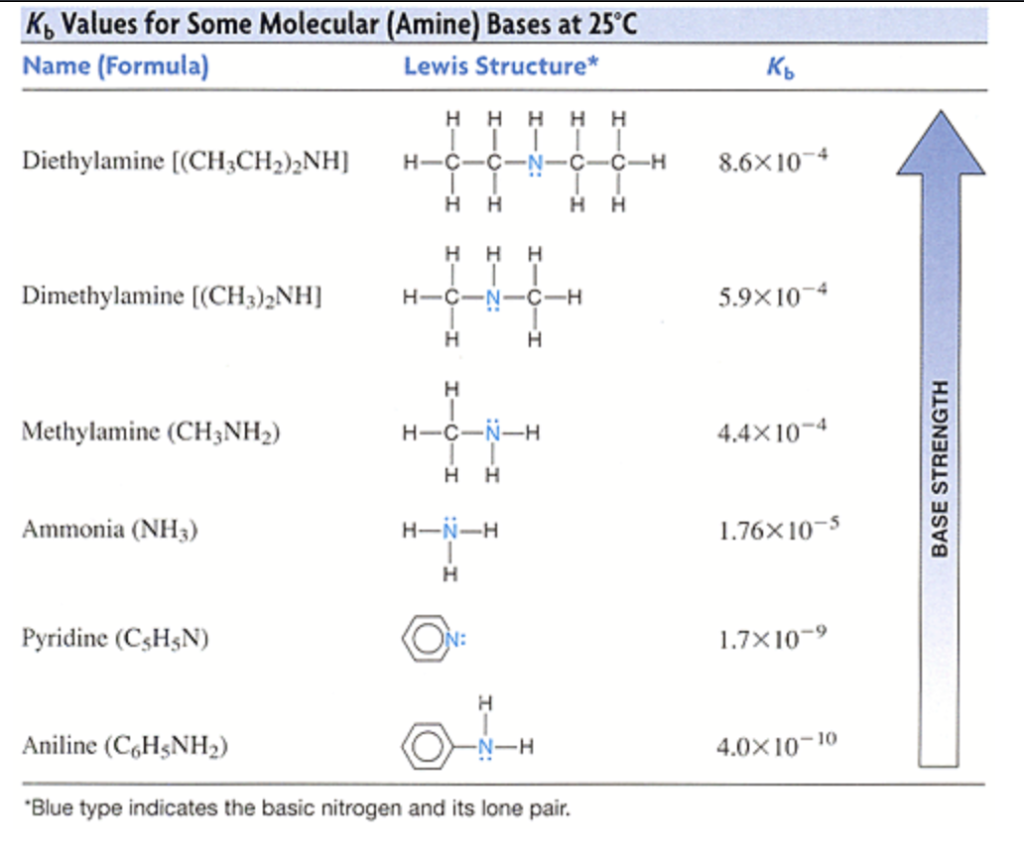
SOLVED: A chemistry graduate student is given 450 mL of 0.10 M diethylamine ((C2H5)2NH) solution. Diethylamine is a weak base with Kb = 1.3x10^-5. What mass of (C2H5)2NHBr should the student dissolve
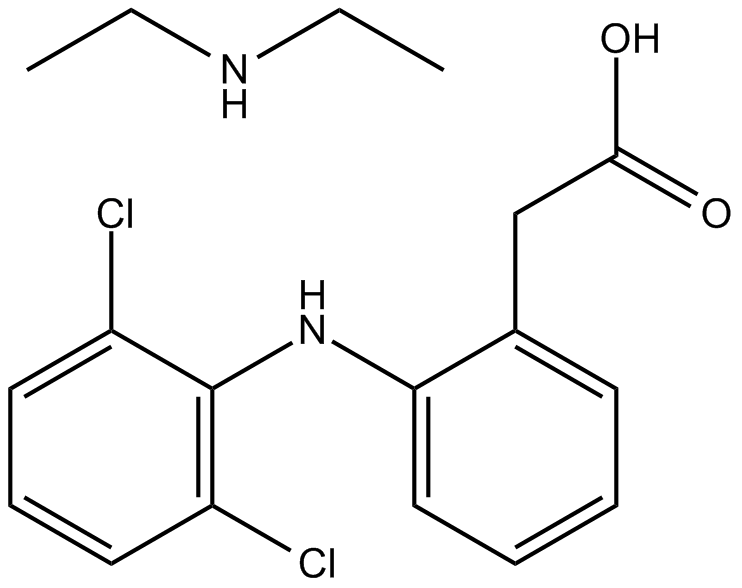
DICLOFENAC DIETHYLAMINE + LINSEED OIL + METHYL SALCILATE + MENTHOL +BENZYL ALCOHOL GEL BASE, ALLEN DALE BIOSCIENCES, Panchkula