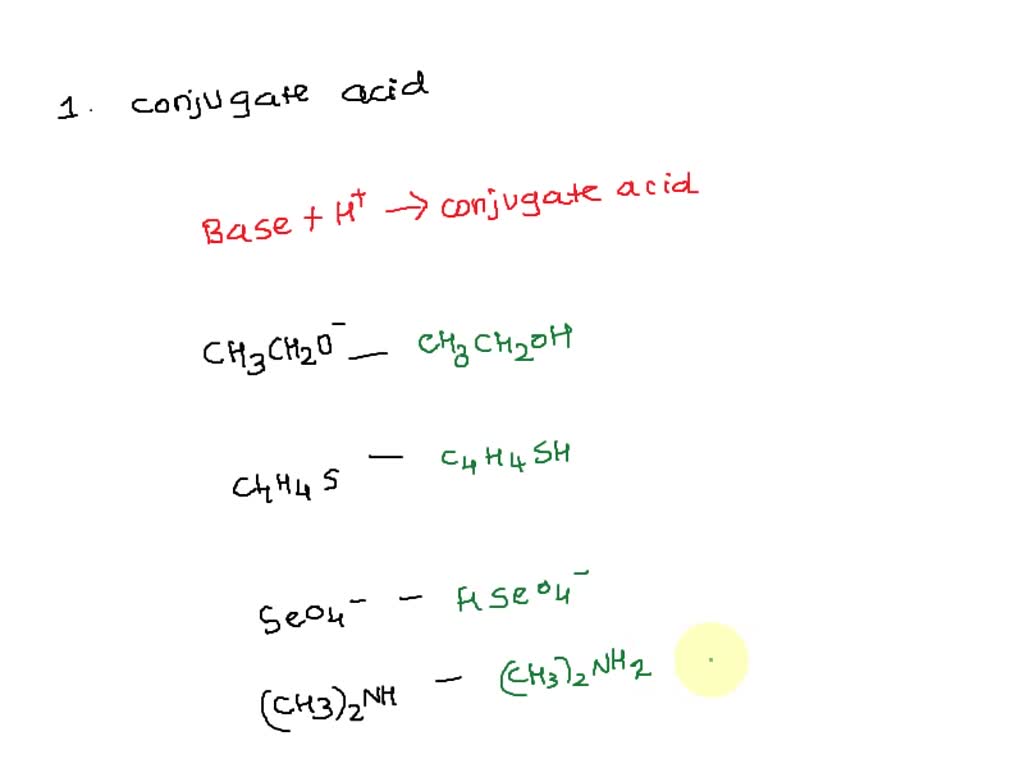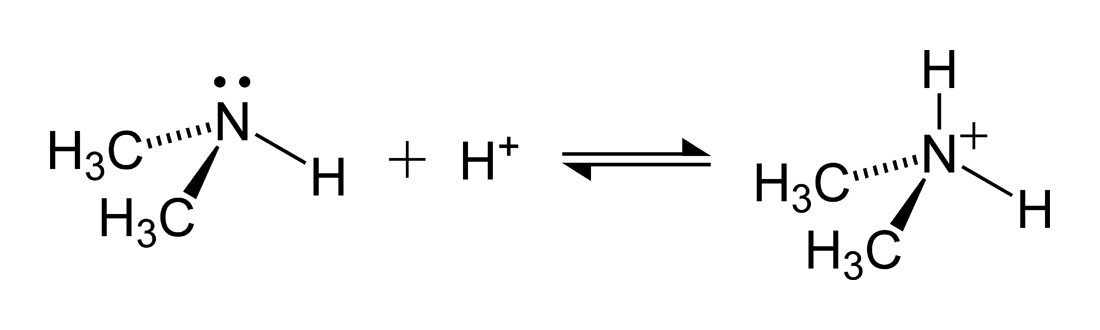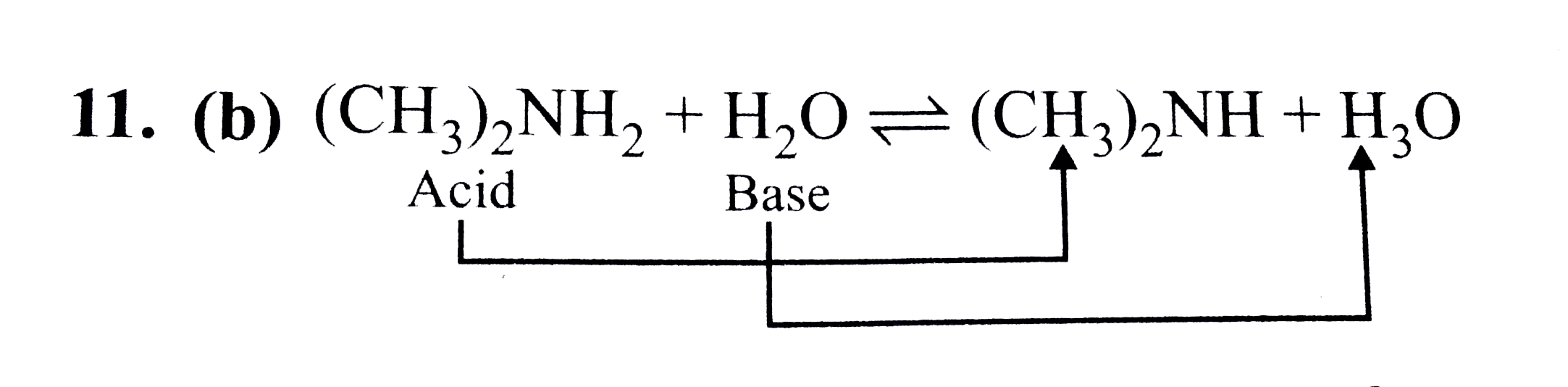Give reasons : (i) (CH3)2NH is more basic than (CH3)3N in an aqueous solution. - Sarthaks eConnect | Largest Online Education Community

SOLVED: 1.) A 21.4 mL sample of 0.271 M dimethylamine, (CH3)2NH, is titrated with 0.372 M nitric acid. After adding 23.5 mL of nitric acid, the pH is . 2.) A 29.4

What is the order of basicity of the following compounds? CH3NH2, (CH3)2NH, (CH3)3N (in protic solvent)

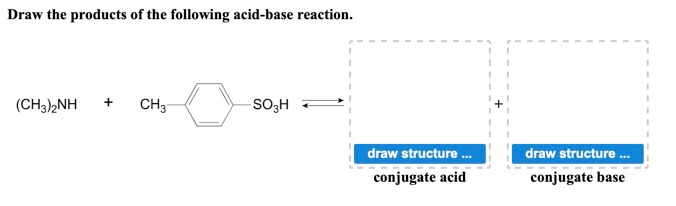
Provide the structure of the conjugate acid and the conjugate base of dimethylamine ((CH3)2NH). | Homework.Study.com